Dear pastry and science enthusiasts,
How many times have you faced a recipe that says, “cook the sugar to the soft-ball stage”? Often, hidden behind these simple words lies a universe of chemical and physical transformations that can make or break a dessert. Sugar is not just a sweetener; it’s a star performer, a building material capable of taking on incredible shapes and textures.
Today, we’re putting sucrose itself under the lens. But we won’t just describe it. We’ll take a journey together, thermometer in hand, to decode its secrets and transform the fear of burnt caramel into pure technical mastery.
Sugar’s Identity Card
- Chemical Name: Sucrose
- Chemical Formula: C₁₂H₂₂O₁₁
- Molecular Composition: It is a disaccharide, the union of two simpler sugars (monosaccharides): one molecule of glucose and one of fructose.
- Main Origin: Sugar beet (Beta vulgaris) and sugarcane (Saccharum officinarum).
- Key Characteristic: It is hygroscopic, meaning it loves water and tends to absorb it from the environment.
A Bit of History (and Science)
Its apparent simplicity hides a fascinating complexity. When we dissolve sugar in water and turn on the heat, we are not just making sweet water. We are orchestrating a series of precise reactions that allow us to build the texture of our dessert.
But for the professionals and the curious who want to go deeper, it’s time to put on the lab coat. Let’s analyze these phenomena as in a true Food Technology Masterclass.
Masterclass – Module 1: The Fundamentals of the Sucrose-Water System
In Technical Terms:
Cooking sugar is a process of concentration by evaporation of an aqueous solution. The behavior of this food matrix is governed by three interconnected variables: Temperature (T), the energy input; Concentration of Soluble Solids (°Brix), the weight percentage of sugar; and Water Activity (aw), the fraction of “free” water available for reactions and microbial growth. A key factor is the acid-catalyzed hydrolysis of sucrose, which splits it into glucose and fructose (invert sugar), molecules that act as powerful anti-crystallization agents by interfering with the formation of the sucrose crystal lattice.
In Simple Terms (but not simplistic):
Let’s imagine our saucepan is a party 🥳. Temperature is the accelerator 🔥. Concentration is how crowded the room is. Water Activity (aw) is the availability of the guests: if all the water is “busy dancing” with the sugar, it’s not available to let in unwanted guests like microbes 💧.
Our anti-crystallization “trick” is hydrolysis. Imagine sucrose molecules as identical blue LEGO® bricks, all easy to stack into an orderly crystal. By adding heat and acid (lemon) 🍋, we break some blue bricks into two different pieces: a red one (glucose) and a yellow one (fructose). Now, try building an orderly wall with different shaped bricks! It’s almost impossible. This “controlled chaos” keeps the syrup smooth and non-gritty.
Masterclass – Module 2: The Rheological Scale and Glass Transition
In Technical Terms:
As concentration increases, we enter the domain of glass transition. The system, once cooled, solidifies into an amorphous (non-crystalline) state. The behavior of this solid is determined by its Glass Transition Temperature (Tgs).
- Soft-Ball Stage (116-121°C / 240-250°F): The system’s Tgs is below room temperature. The product therefore behaves like a “rubbery,” deformable solid.
- Hard-Ball Stage (126-135°C / 260-275°F): The system’s Tgs is above room temperature. The product behaves like a “glassy,” rigid, and brittle solid.
In Simple Terms (but not simplistic):
The key concept is sugar’s “molecular thermostat” (Tgs). Think of a chocolate bar 🍫: in the fridge, it’s hard and brittle (glassy state); in the sun, it’s soft and pliable (rubbery state). The temperature at which this change occurs is its Tgs. We, by controlling the water, get to program this thermostat!
- Soft-Ball Stage: We program the Tgs to a low value (e.g., 10°C / 50°F). At room temperature (20°C / 68°F), the syrup is above its Tgs, so it stays soft.
- Hard-Ball Stage: We program the Tgs to a high value (e.g., 40°C / 104°F). At room temperature, the syrup is below its Tgs, so it becomes hard like glass.
Masterclass – Module 3: Pyrolysis and the Birth of Caramel
In Technical Terms:
Above 140-150°C (284-302°F), water is almost absent, and the pyrolysis of sucrose begins. This is a non-enzymatic thermal degradation. The molecule undergoes fragmentation reactions (generating volatile aromatic compounds like furans and maltol) and polymerization reactions (creating non-volatile colored macromolecules: caramelans, caramelens, and caramelins). The combination of these new molecules defines the color and flavor profile of caramel.
In Simple Terms (but not simplistic):
What happens when sugar becomes caramel? Imagine overheating our LEGO® bricks until they melt 🫠.
- They Break Apart (Fragmentation): The high heat shatters the sugar molecules into tiny pieces. Some of the lightest ones fly away: these are the molecules we perceive as the aroma and flavor of caramel.
- They Recombine Randomly (Polymerization): The pieces left in the pot, glowing hot and reactive, bind together in new, complex ways, forming bigger and darker clumps. These new mega-molecules are what give caramel its color. Caramel is no longer sugar; it’s a whole new family of substances created by fire!
The Practical Guide to the Sugar Cooking Stages
With this scientific awareness, let’s return to our saucepan. Here is the operational guide to recognize and use each stage.
1. Thread Stage (105-112°C / 221-234°F)
A thin thread forms when lifted. Ideal for syrups and fruit preserves.
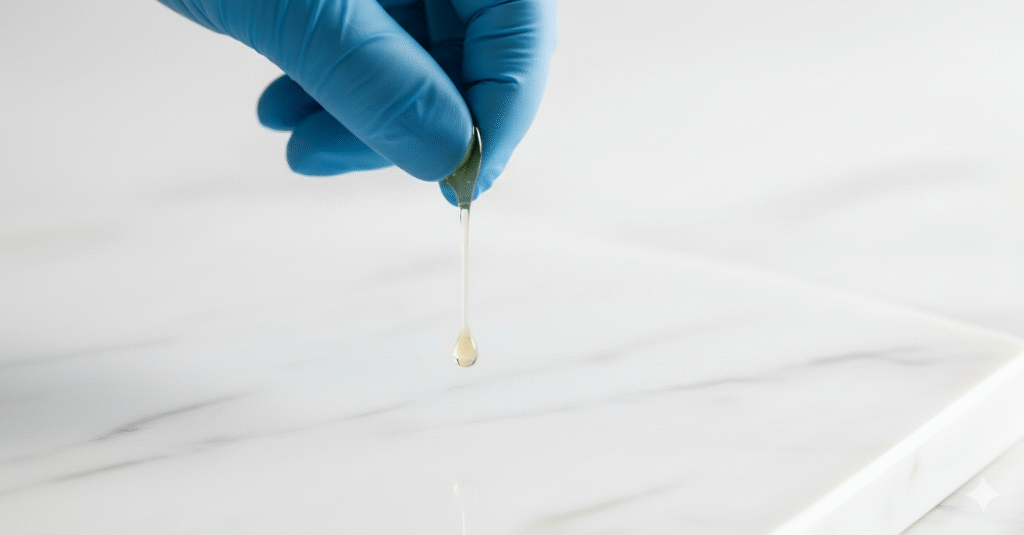
2. Soft-Ball Stage (116-121°C / 240-250°F)
A drop in cold water forms a soft, pliable ball. This is the crucial stage for Italian meringue, soft nougat, and fudge.
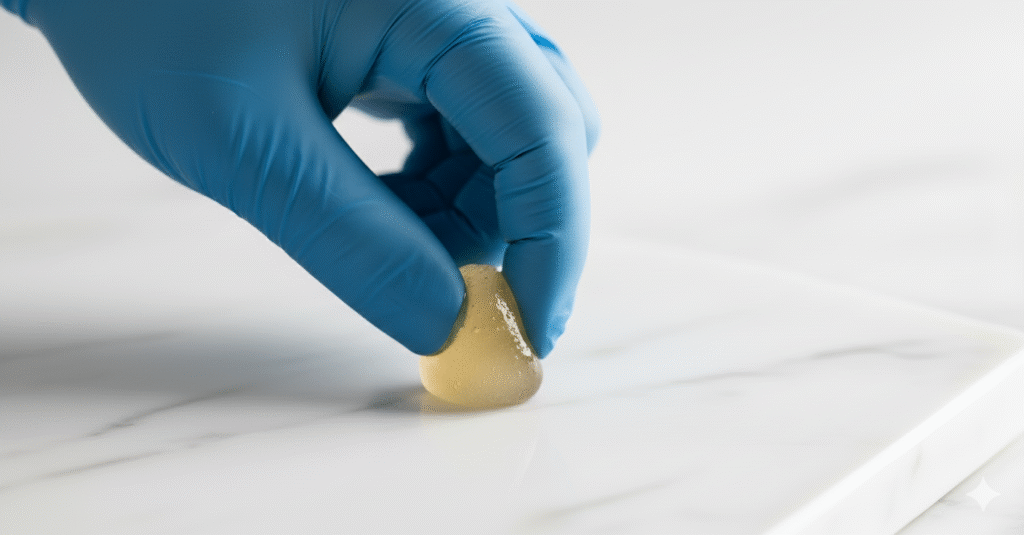
3. Hard-Ball Stage (126-135°C / 260-275°F)
A drop in cold water forms a hard ball. Perfect for hard nougat and hard candies.

4. Light Caramel Stage (160-165°C / 320-329°F)
The sugar takes on a light amber hue and fragrance. Used for crème caramel, pralines, and spun sugar decorations.

5. Dark Caramel Stage (166-175°C / 330-347°F)
The color deepens to an intense mahogany, with toasted notes. Suitable for complex sauces and balancing very sweet desserts.

The Biologist Pastry Chef’s Tip
The number one enemy is premature crystallization. My 3-step anti-crystallization strategy:
- Cleanliness is Prevention: Use a heavy-bottomed, perfectly clean saucepan.
- The Clean Edge Technique: While the syrup cooks, dip a pastry brush in water and wipe the inside edges of the pot. This dissolves any stray crystals, neutralizing the risk of a chain reaction.
- The Chemical Assist: A few drops of lemon juice at the beginning promotes sugar inversion, acting as a natural insurance policy against crystallization.
Conclusion
You now have the complete map to navigate the world of cooked sugar, from its molecular identity to its most delicious application. Pastry is not just recipes; it’s a deep understanding of your ingredients.
Which cooking stage fascinates you the most? Let me know in the comments!
Until the next discovery,
Katia Oldani
Biologist Pastry Chef
Click Here and Visit My YouTube Channel
For any information or contact, CLICK HERE
#IngredientsUnderTheLens #BiologistPastryChef #PastryScience #Sugar #Caramel #PastryTechniques #FoodScience #KatiaOldani


